Cannabinoid Agonist Clinical Trial Pipeline Appears Robust With 20+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The cannabinoid agonist market is gaining momentum due to expanding medical cannabis legalization and growing clinical validation for its use in chronic pain, epilepsy, and inflammatory conditions. Increasing patient demand for natural therapies and advancements in receptor biology are accelerating innovation. Pharmaceutical investments and diverse clinical trials are further shaping this evolving space.
New York, USA, July 10, 2025 (GLOBE NEWSWIRE) -- Cannabinoid Agonist Clinical Trial Pipeline Appears Robust With 20+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The cannabinoid agonist market is gaining momentum due to expanding medical cannabis legalization and growing clinical validation for its use in chronic pain, epilepsy, and inflammatory conditions. Increasing patient demand for natural therapies and advancements in receptor biology are accelerating innovation. Pharmaceutical investments and diverse clinical trials are further shaping this evolving space.
DelveInsight’s 'Cannabinoid Agonist Pipeline Insight 2025' report provides comprehensive global coverage of pipeline cannabinoid agonists in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space, and the future growth potential of the cannabinoid agonist pipeline domain.
Key Takeaways from the Cannabinoid Agonist Pipeline Report
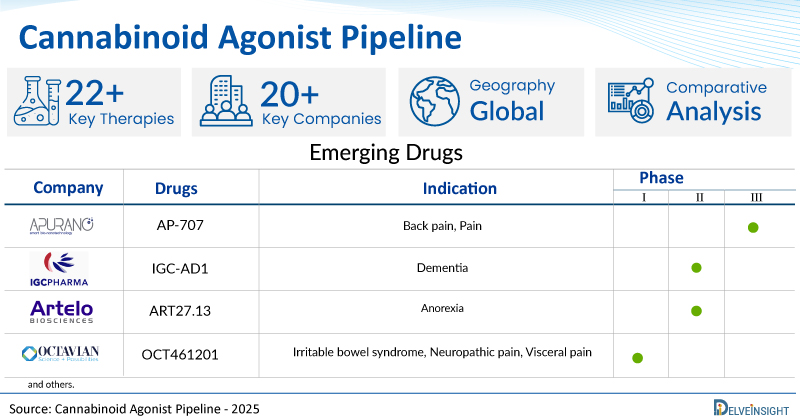
- DelveInsight’s cannabinoid agonist pipeline report depicts a robust space with 20+ active players working to develop 22+ pipeline cannabinoid agonists.
- Key cannabinoid agonist companies such as Apurano Pharmaceuticals, IGC Pharma, Inc., Artelo Biosciences, Inc., NeuroTherapia, Oxford Cannabinoid Technologies Holdings, SciSparc, Mira Pharmaceuticals, InMed Pharmaceuticals, Corbus Pharmaceuticals, and others are evaluating new cannabinoid agonist drugs to improve the treatment landscape.
- Promising pipeline cannabinoid agonists such as AP-707, IGC-AD1, ART27.13, NTRX 07, OCT461201, SCI 160, MIRA-55, INM 901, CRB 913, and others are under different phases of cannabinoid agonist clinical trials.
- In July 2025, MIRA Pharmaceuticals announced positive preclinical data demonstrating that Mira-55, the Company’s proprietary non-psychotropic marijuana analog, delivered morphine-comparable pain relief in a validated model of inflammatory pain-without causing local inflammation.
- In June 2025, InMed Pharmaceuticals announced new preclinical data demonstrating that INM-901 significantly reduces inflammation in ex vivo models of neuroinflammation, further supporting its potential as a therapeutic candidate in Alzheimer’s disease.
- In March 2025, Corbus Pharmaceuticals Holdings announced the dosing of the first subject in the single ascending dose / multiple ascending dose (SAD/MAD) portion of the Phase I trial of CRB-913 cannabinoid type-1 (CB1) receptor inverse agonist drug for the treatment of obesity. The study is being conducted in the United States under an open IND.
- In March 2025, IGC Pharma announced additional positive interim results from its ongoing Phase II clinical trial on IGC-AD1, an investigational treatment for agitation in dementia due to Alzheimer's disease.
- In February 2025, NeuroTherapia announced it had received approval for its Phase II clinical trial from the European Medicines Agency (EMA). NTRX-07, the Company's lead molecule, will be administered to Alzheimer's disease (AD) participants for 28 days in this double-masked, randomized clinical trial.
- In December 2024, Artelo Biosciences announced the presentation of preliminary data on ART27.13, the Company’s benzimidazole derivative, being studied for cancer-related anorexia.
- In October 2024, Oxford Cannabinoid Technologies Holdings announced that dosing of all the cohorts of the Phase I, single ascending dose study for OCT461201 has been successfully completed. No safety or tolerability concerns were exhibited with any dose tested.
Request a sample and discover the recent advances in cannabinoid agonist drugs @ Cannabinoid Agonist Pipeline Report
The cannabinoid agonist pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage cannabinoid agonist drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the cannabinoid agonist clinical trial landscape.
Cannabinoid Agonist Overview
Cannabinoid agonists are substances that activate the cannabinoid receptors CB1 and CB2, which are integral to the endocannabinoid system. These agents can be derived from natural sources, produced within the body, or synthetically manufactured. They are being investigated and utilized for a wide range of therapeutic purposes, including pain relief, appetite enhancement, anti-inflammatory effects, and protection of nerve cells. The rising prevalence of chronic conditions such as cancer, epilepsy, neurodegenerative disorders, and persistent pain has led to growing interest in developing cannabinoid-based treatments.
Structurally, cannabinoid agonists often contain terpene and phenolic elements, which are common in both plant-derived cannabinoids and synthetic variants. Endocannabinoids such as anandamide are characterized by a fatty acid framework with an amide group and extended carbon chains. Phytocannabinoids typically feature a benzopyran core fused with a terpene moiety, whereas synthetic cannabinoids tend to incorporate altered aromatic structures to enhance receptor specificity. These chemical differences influence how effectively these compounds bind to and activate cannabinoid receptors, thereby affecting various bodily functions.
Cannabinoid receptors play distinct roles:
- CB1 receptors are mainly located in the brain and central nervous system and are involved in regulating mood, memory, pain sensation, and appetite.
- CB2 receptors are predominantly found in immune cells and peripheral tissues, contributing to immune response and inflammation control.
Beyond these targets, some cannabinoid agonists also act on other systems, such as TRPV1 receptors and serotonin pathways, broadening their potential medical applications.
Due to their widespread biological effects, cannabinoid agonists are being used in numerous clinical areas. They are particularly effective in managing chronic and neuropathic pain, reducing inflammation in autoimmune conditions like rheumatoid arthritis, and treating neurological issues such as epilepsy, multiple sclerosis, and Parkinson’s disease. In mental health, they show promise for treating anxiety, depression, and PTSD. In oncology and HIV/AIDS care, they help control chemotherapy-related nausea and stimulate appetite. Their ability to protect nerve cells also makes them candidates for treating Alzheimer's and other neurodegenerative illnesses.
Find out more about cannabinoid agonist drugs @ Cannabinoid Agonist Analysis
A snapshot of the Pipeline Cannabinoid Agonist Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| AP-707 | Apurano Pharmaceuticals | III | Back pain, Pain | Sublingual |
| IGC-AD1 | IGC Pharma, INC | II | Dementia | Oral |
| ART27.13 | Artelo Biosciences, Inc. | II | Anorexia | Oral |
| OCT461201 | AskAt/Octavian Therapeutics | I | Irritable bowel syndrome, Neuropathic pain, Visceral pain | Oral |
| NTRX 07 | NeuroTherapia | I | Alzheimer's disease | Oral |
Learn more about the emerging cannabinoid agonist @ Cannabinoid Agonist Clinical Trials
Cannabinoid Agonist Therapeutics Assessment
The cannabinoid agonist pipeline report proffers an integral view of the emerging cannabinoid agonist segmented by stage, product type, molecule type, and route of administration.
Scope of the Cannabinoid Agonist Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Key Cannabinoid Agonist Companies: Apurano Pharmaceuticals, IGC Pharma, Inc., Artelo Biosciences, Inc., NeuroTherapia, Oxford Cannabinoid Technologies Holdings, SciSparc, Mira Pharmaceuticals, InMed Pharmaceuticals, Corbus Pharmaceuticals, and others.
- Key Cannabinoid Agonist Pipeline Therapies: AP-707, IGC-AD1, ART27.13, NTRX 07, OCT461201, SCI 160, MIRA-55, INM 901, CRB 913, and others.
Dive deep into rich insights for new cannabinoid agonists, visit @ Cannabinoid Agonist Drugs
Table of Contents
| 1. | Cannabinoid Agonist Pipeline Report Introduction |
| 2. | Cannabinoid Agonist Pipeline Report Executive Summary |
| 3. | Cannabinoid Agonist Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Cannabinoid Agonist Clinical Trial Therapeutics |
| 6. | Cannabinoid Agonist Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Cannabinoid Agonist Pipeline: Late-Stage Products (Phase III) |
| 8. | Cannabinoid Agonist Pipeline: Mid-Stage Products (Phase II) |
| 9. | Cannabinoid Agonist Pipeline: Early-Stage Products (Phase I) |
| 10. | Cannabinoid Agonist Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Cannabinoid Agonist Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Cannabinoid Agonist Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the cannabinoid agonist pipeline therapeutics, reach out @ Cannabinoid Agonist Therapeutics
Related Reports
Alzheimer's Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Alzheimer's disease companies, including AB Science, Alzheon Inc., AriBio Co., Ltd., AgeneBio, Inc., Anavex Life Sciences Corp., Annovis Bio, Inc., Cerecin, BioVie, Cassava Sciences, Novo Nordisk, Eli Lilly, Neurim Pharmaceuticals, Suven Life Sciences, Bristol Myers Squibb, Karuna Therapeutics, T3D Therapeutics, Inc., Lexeo Therapeutics, Axsome Therapeutics, Inc., Araclon Biotech S.L., Eisai Co., Ltd., TauRx Therapeutics, TrueBinding, Inc., AC Immune SA, Johnson & Johnson, Longeveron Inc., Vaccinex Inc., IGC Pharma LLC, among others.
Alzheimer's Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Alzheimer's Disease companies, including Biogen, AZTherapies, Cerecin, Neurotrope, Synaptogenix, INmune Bio, Cassava Sciences, EIP Pharma, Neuraly, AB Science, Cortexyme, Anavex Life Sciences, Athira Pharma, Time Therapeutics, Denali Therapeutics Inc., Alector Inc., Lexeo Therapeutics, TrueBinding, Inc., Vaccinex Inc., Annovis Bio Inc., Eisai Inc., Hoffmann-La Roche, Ionis Pharmaceuticals, Inc., Otsuka Pharmaceutical Co., Ltd., Cognition Therapeutics, Merck Sharp & Dohme LLC, ImmunoBrain Checkpoint, AbbVie, AriBio Co., Ltd., Oryzon Genomics S.A., Eli Lilly and Company, Neurokine Therapeutics, Excelsior, Seelos Therapeutics, Inc., Janssen Research & Development, LLC, Shanghai Hengrui Pharmaceutical Co., Ltd., reMYND, Alzinova AB, VTBIO Co. LTD, BioVie Inc., Prothena Corporation plc, Coya Therapeutics, Inc., among others.
Parkinson's Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Parkinson’s disease companies, including UCB Biopharma SRL, Novartis, Annovis Bio, Supernus Pharmaceuticals, Inc., Britannia Pharmaceutical, Pharma Two B, Mitsubishi Tanabe Pharma (NeuroDerm), AbbVie, Cerevel Therapeutics, Cerevance, among others.
Parkinson's Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Parkinson's disease companies, including Cerevel Therapeutics, Inhibikase Therapeutics, Neuraly, Peptron, Biogen, Roche, Brain Neurotherapy Bio, Inc., Modag, Annovis Bio Inc., BioVie Inc., United Neuroscience Ltd., Luye Pharma Group, AbbVie, UCB Biopharma SRL, InnoMedica Schweiz AG, Integrative Research Laboratories AB, H. Lundbeck A/S, Shanghai WD Pharmaceutical Co., Ltd., Cerevance Beta, Inc., Nobilis Therapeutics Inc., BlueRock Therapeutics, Taiwan Mitochondrion Applied Technology Co., Ltd., among others.
Parkinson's Disease Psychosis Market
Parkinson's Disease Psychosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Parkinson’s disease psychosis companies, including Sumitomo Pharma America Inc., Vanda Pharmaceuticals, Acadia Pharmaceuticals Inc., Otsuka America Pharmaceutical, Lundbeck LLC, Jazz Pharmaceuticals, Alkahest Inc., Sandoz, Sio Gene Therapies, Axovant Sciences Ltd, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +91-9650213330 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
